Introduction:
Since the beginning of 2020, numerous medications have been nominated to control COVID-19. According to some studies, formoterol may have a potential role in COVID-19 as an adjunctive therapy (1). This randomized controlled, open-label trial, was aimed to compare the value of inhaled formoterol on the respiratory system improvement in outpatients with confirmed COVID-19.
Methods:
From 23 March to 20 April 2020, in Tehran and Rasht, covid19 patients aged 18 years or older with respiratory symptoms and O2 saturation more than 93%, were randomly assigned to receive standard regimen (according national protocol) with or without formoterol (Bronchovent 12mcg, Cyprus, Medochemie).
Results:
A total of 60 patients (formoterol: N=35, mean age: 47.6±11.1 years, 45.7% female; control: N=25, 51.9±12 years, 36% female) were enrolled with no significant difference in the baseline characteristics. In the ten-day follow-up, total respiratory symptom-relief was reported in 68.6. % and 36% in the formoterol and control group, P=0.003. Moreover, the rate of fever, cough, and dyspnea was 97.1%, 100%, and 65.7% in the formoterol group which after 10 days decreased to 8.6%, 31.4%, 11.4%. In the control group, these symptoms were 92%, 100%, and 60% respectively which reached 12%, 64%, and 36% within 10 days.
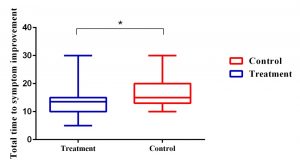
Intergroup analysis showed that formoterol compared to the control group could not only significantly improve cough (P = 0.01), and Dyspnea (P = 0.02) but could also reduce the duration of total respiratory symptoms. (Graph 1)
Conclusion:
Inhaled formoterol can be an effective additive treatment in COVID-19.
References:
Giovanni Ghirga, Potential treatment for Covid-19 from asthma armamentarium, BMJ 2020;368:m1252,